Doctors should avoid giving steroids to COVID-19 patients and recommend tuberculosis tests if their cough persists, according to the government’s revised clinical guidelines for coronavirus treatment, released just days after the task force’s chief expressed regret for the drug’s overuse during the second wave.
According to the amended guidelines, medications such as steroids might raise the risk of a secondary infection such as invasive mucormycosis or ‘black fungus’ when administered too early, at a greater dose, or for a longer period of time than necessary.
The guidelines stated that if a cough persists for more than two to three weeks, patients should be checked for TB and other illnesses. They also specified the doses for the medications if necessary for three types of infections – “mild, moderate, and severe.”
Dr V K Paul, NITI Aayog Member (Health) and Chief of the Covid Task Force highlighted worries about the “overuse and abuse” of medications like steroids at a news conference last week.

Upper respiratory tract symptoms without shortness of breath or hypoxia have been classified as a moderate illness, and home isolation and care have been indicated.
Mild Covid patients should get medical assistance if they have trouble breathing, a high temperature, or a severe cough that lasts more than five days.
Those who have shortness of breath and variable oxygen saturation of 90-93 per cent might be classified as intermediate cases. Such patients should be provided with oxygen.
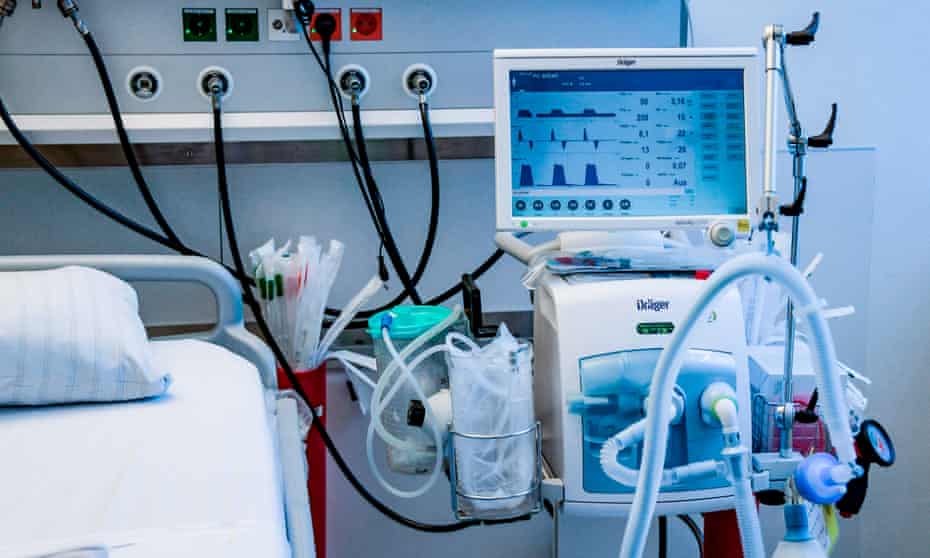
Respiratory rate greater than 30 beats per minute, dyspnea, or oxygen saturation less than 90% on room air should be considered a serious illness, according to the report, and such patients should be sent to an ICU since they would require respiratory assistance.
Such patients should be placed on mechanical ventilation. If the work of breathing is modest, non-invasive ventilation (NIV) – helmet or face mask interface depending on availability—may be explored in individuals with growing oxygen requirements.
The new recommendations continue to advise patients with “moderate to severe” illness and no renal or hepatic impairment to seek emergency use authorization (EUA) or off-label use of remdesivir within 10 days of the beginning of any symptom.
It cautioned against using the medicine on patients who are not on oxygen assistance or who are in an in-home environment.
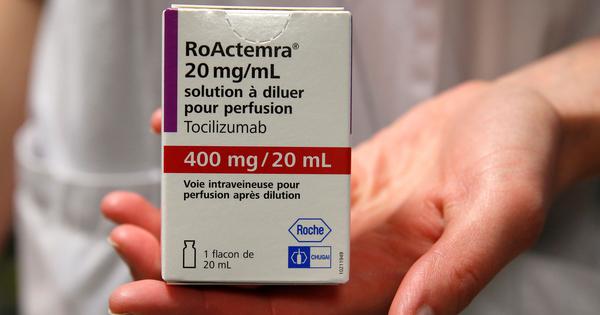
EUA or off-label use of the tocilizumab medicine may also be explored in the case of severe disease, ideally within 24 to 48 hours after the beginning of the severe disease or ICU admission, according to the recommendations.
Tocilizumab may be evaluated for individuals with considerably elevated inflammatory markers who are not improving despite the administration of steroids and have no current bacterial, fungal, or tubercular infection, according to the researchers.
The guidelines state that those over the age of 60, as well as those with cardiovascular disease, hypertension and coronary artery disease, diabetes mellitus, and other immunocompromised states such as HIV, active tuberculosis, chronic lung, kidney, or liver disease, cerebrovascular disease, or obesity, are at high risk for severe disease and mortality.
Also Checkout: In a historical-first transplant, a man receives a genetically engineered pig heart
















